The FDA announced late Thursday that it would issue an emergency use authorization (EUA) for Moderna’s COVID vaccine, bringing our immunization arsenal to a total of two. This vaccine is similar to the Pfizer/BioNTech one that got an EUA last week.
It’s great news that we have two vaccines now, even though they are similar, because this means two companies are now able to make and distribute doses for the vaccine. The supply is still very limited, so only a few people have managed to get the vaccine so far — mainly doctors and other healthcare workers.
[referenced id=”1037714″ url=”https://www.lifehacker.com.au/2020/11/who-will-get-the-vaccine-first__trashed/” thumb=”” title=”Who Will Get the Vaccine First?” excerpt=”One COVID-19 vaccine is already under review for possible emergency authorization in December; its competitors are close behind. Once we have a vaccine — or maybe several — it will be a while until there are enough doses for everybody. The CDC is working on a plan to prioritise certain…”]
So two vaccines are approved?
Not approved, “emergency use authorised.” This is basically a temporary approval for use while the pandemic is raging. The FDA expects both companies to continue doing studies and to apply for full approval when they can.
[referenced id=”1040203″ url=”https://www.lifehacker.com.au/2020/12/how-they-made-a-vaccine-so-fast/” thumb=”https://www.gizmodo.com.au/wp-content/uploads/sites/4/2020/12/16/vaccine-coronavirus-300×154.png” title=”How They Made a Vaccine So Fast” excerpt=”Vaccine development takes time. Earlier this autumn, we learned that the previous record for vaccine development was four years from sample to approval, and that we might not see a COVID vaccine for years, if at all. But here we are, barely one year past the discovery of the novel…”]
The difference is important because an EUA can be quickly revoked if there is new information that gives reason to believe it’s not safe enough or effective enough. It also only applies during emergency conditions, so if COVID vaccines are to become part of our new normal, they’ll eventually have to go through all the same paperwork as any other vaccine.
Also, as I write, the second EUA hasn’t actually been granted. When the Pfizer vaccine was up for consideration last week, the FDA’s advisory panel met on Thursday, and the FDA cranked out the official paperwork by Friday night. They don’t seem to be in as much of a rush this time, so it might become official over the weekend rather than today.
Are the two vaccines basically the same?
Pretty much. They both contain an mRNA coding for the coronavirus’s spike protein. That mRNA is coated in a lipid nanoparticle, sort of like a tiny soap bubble, and then thousands of those particles are suspended in a solution of sugars, salts, and/or pH-stabilizing buffers. That’s it.
[referenced id=”1040421″ url=”https://www.lifehacker.com.au/2020/12/how-mrna-vaccines-work/” thumb=”https://www.gizmodo.com.au/wp-content/uploads/sites/4/2020/12/17/i3izugpc9sze7tpxs09g-300×169.jpg” title=”How mRNA Vaccines Work” excerpt=”The first COVID vaccine to be rolled out in the U.S., the one from Pfizer and BioNTech, is an mRNA vaccine. The second one probably will be too: Moderna’s vaccine is up for consideration this week. We’ve never had an mRNA vaccine in common use before, so you’re not alone…”]
Once injected, our cells use the mRNAs to make the spike protein, and then our immune system reacts to the spike protein, giving us immunity. That immune system response can give us a sore arm and sometimes fever, chills or fatigue for a day or two.
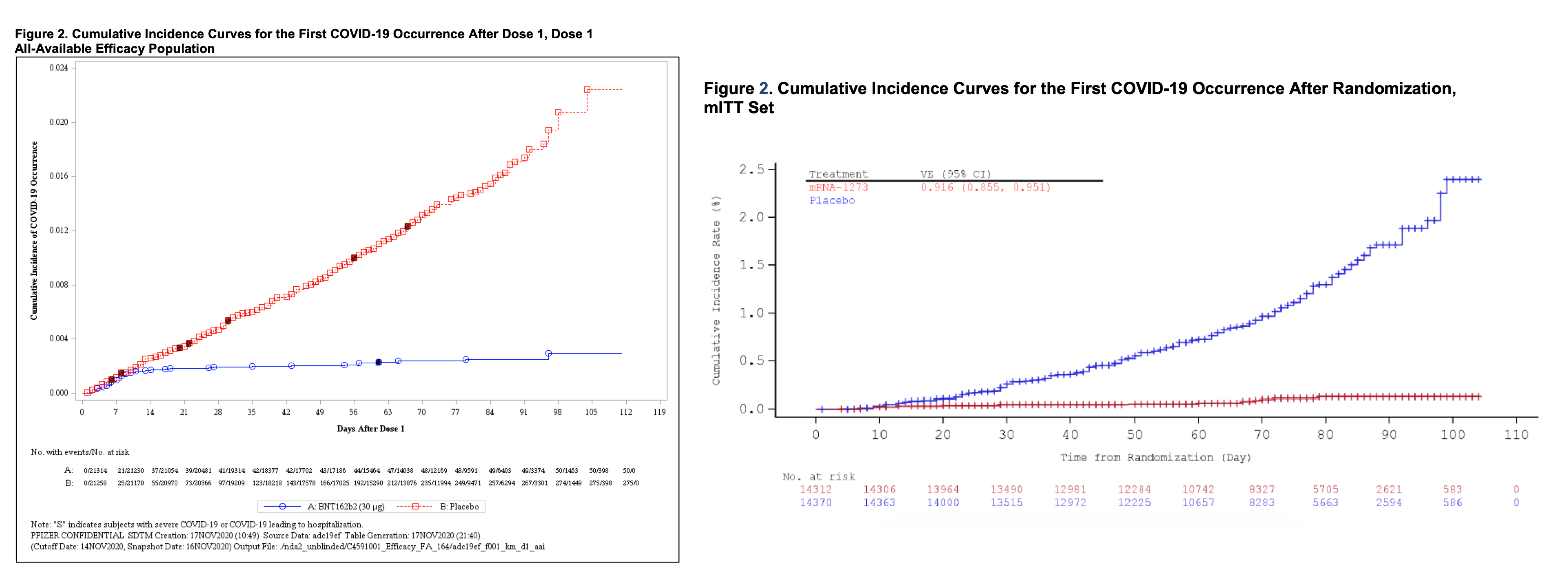
Both vaccines turned out to be around 95% effective in trials. Both vaccines are given as a two-dose series, but cumulative incidence charts, shown above, indicate that protection begins about 14 days after the first dose. After that point, people in the control groups continued to get COVID (increasing line), while very few cases showed up in the vaccine groups (flat line).
What are the differences?
The two doses of the Pfizer/BioNTech vaccine are given three weeks apart; the doses of the Moderna vaccine are given four weeks apart.
The side effects of both are similar, with those from the Moderna vaccine possibly being stronger. Temporary swelling of armpit lymph nodes was more common with this vaccine than with the Pfizer one. Still, side effects of both are mild to moderate in most people.
[referenced id=”1039089″ url=”https://www.lifehacker.com.au/2020/12/what-will-it-feel-like-to-get-a-covid-vaccine/” thumb=”https://www.gizmodo.com.au/wp-content/uploads/sites/4/2020/12/05/ycailx4ppltv2ct1v6ax-300×169.jpg” title=”What Will It Feel Like to Get a COVID Vaccine?” excerpt=”Every vaccine has its side effects, but most are mild and rare. If you’ve ever had a sore arm after a flu shot, or even a mild headache or fever, you’ve experienced these. The upcoming coronavirus vaccines will have side effects, too, and they might be a little more severe….”]
The Pfizer/BioNTech vaccine is authorised for people aged 16 and up; the Moderna vaccine is for people aged 18 and up.
Storage is different, too. The Moderna vaccine can be stored at normal freezer temperatures. The Pfizer/BioNTech vaccine needs to stay ultra-cold, so it’s being distributed in thermos-like packages that each contain 975 doses. The packaging keeps the vaccine cold enough, but the large number of doses means there’s no easy way to provide this vaccine to smaller clinics. Going forward, it’s possible that rural and remote areas might be more likely to use the Moderna vaccine.
Can I mix and match?
Both vaccines require two doses, but there haven’t been any trials that test what happens if you get one dose of each. So your second dose should be the same kind of vaccine that you got for your first dose.
Manufacturers plan to give people a wallet card as a reminder of which vaccine they got and when they’re due back for the second dose. Keep this with you, or take a photo with your phone.
Each vaccine has a separate code for medical records, so if you forget which you got, it should be possible to check with your doctor (or the clinic where you got the vaccine) to find out. These separate codes also mean that it will be easier for studies of medical records to tell if there are side effects or other issues that correlate with one vaccine versus another.
Leave a Reply
You must be logged in to post a comment.